Gold Atom Structure
Gold Atom Structure. 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. And for gold, each energy level will hold: 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
Nejchladnější 3d Render Of Atom Structure Of Gold Isolated Over White Background Protons Are Represented As Red Spheres Neutron As Yellow Spheres Electrons As Blu Stock Photo Alamy
79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (blue).Each orbital can hold a certain amount of electrons;
79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 79 electrons (white) successively occupy available electron shells (rings). 79), the most common isotope of this element. It has a melting point of 1064 degrees celsius. These electrons will exist in determined orbitals around the nucleus. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... The nucleus consists of 79 protons (red) and 118 neutrons (blue). 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Each orbital can hold a certain amount of electrons; These electrons will exist in determined orbitals around the nucleus... 79 electrons (white) successively occupy available electron shells (rings).

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (blue). 2, 8, 18, 32, 18, 1. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. It has a melting point of 1064 degrees celsius.

These electrons will exist in determined orbitals around the nucleus.. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. Each orbital can hold a certain amount of electrons; 2, 8, 18, 32, 18, 1. 79), the most common isotope of this element. And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. It has a melting point of 1064 degrees celsius.. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.
The nucleus consists of 79 protons (red) and 118 neutrons (orange).. The nucleus consists of 79 protons (red) and 118 neutrons (blue). 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons; Atomic structure of gold includes atomic number, atomic weight, electron configuration 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). And for gold, each energy level will hold: It has a melting point of 1064 degrees celsius.. The nucleus consists of 79 protons (red) and 118 neutrons (blue).

79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. 79 electrons (white) successively occupy available electron shells (rings). And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1. Atomic structure of gold includes atomic number, atomic weight, electron configuration It has a melting point of 1064 degrees celsius. 2, 8, 18, 32, 18, 1.

And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Atomic structure of gold includes atomic number, atomic weight, electron configuration 79 electrons (white) successively occupy available electron shells (rings)... It has a melting point of 1064 degrees celsius.

79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. Atomic structure of gold includes atomic number, atomic weight, electron configuration 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. These electrons will exist in determined orbitals around the nucleus. And for gold, each energy level will hold:

79 electrons (white) successively occupy available electron shells (rings). It has a melting point of 1064 degrees celsius... The nucleus consists of 79 protons (red) and 118 neutrons (orange).

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus... The nucleus consists of 79 protons (red) and 118 neutrons (blue). 79), the most common isotope of this element. And for gold, each energy level will hold: Atomic structure of gold includes atomic number, atomic weight, electron configuration Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 79 electrons (white) successively occupy available electron shells (rings). It has a melting point of 1064 degrees celsius. These electrons will exist in determined orbitals around the nucleus. These electrons will exist in determined orbitals around the nucleus.
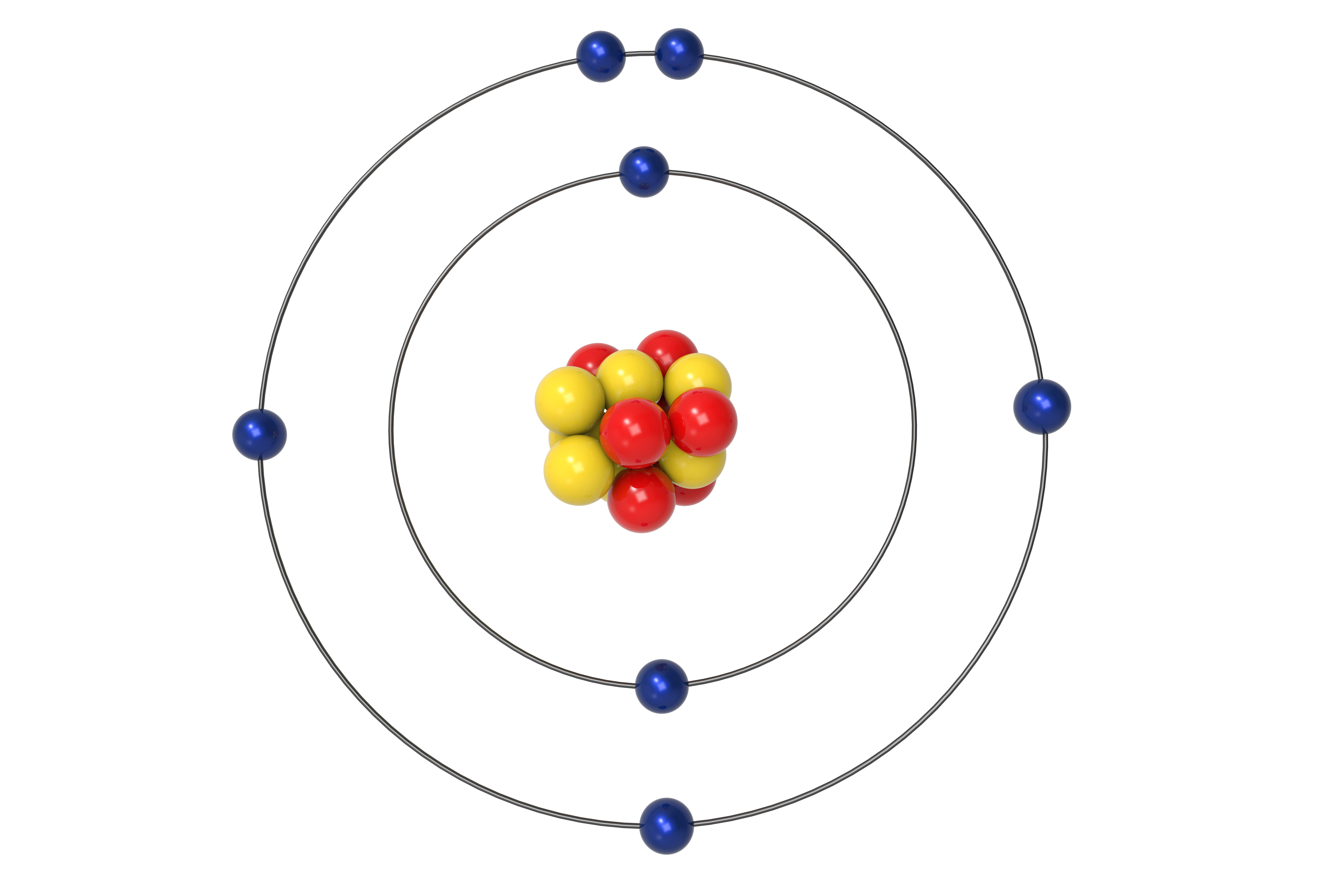
79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). It has a melting point of 1064 degrees celsius... 2, 8, 18, 32, 18, 1.

79 electrons (white) successively occupy available electron shells (rings). 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus.. 79), the most common isotope of this element.

The nucleus consists of 79 protons (red) and 118 neutrons (blue). 79 electrons (white) successively occupy available electron shells (rings).. Each orbital can hold a certain amount of electrons;

These electrons will exist in determined orbitals around the nucleus... . And for gold, each energy level will hold:

Atomic structure of gold includes atomic number, atomic weight, electron configuration 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). It has a melting point of 1064 degrees celsius. Atomic structure of gold includes atomic number, atomic weight, electron configuration.. Atomic structure of gold includes atomic number, atomic weight, electron configuration
Atomic structure of gold includes atomic number, atomic weight, electron configuration Atomic structure of gold includes atomic number, atomic weight, electron configuration. These electrons will exist in determined orbitals around the nucleus.

79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Each orbital can hold a certain amount of electrons; Atomic structure of gold includes atomic number, atomic weight, electron configuration And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus. It has a melting point of 1064 degrees celsius. 79), the most common isotope of this element. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Atomic structure of gold includes atomic number, atomic weight, electron configuration.. 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (blue).

The nucleus consists of 79 protons (red) and 118 neutrons (blue). The nucleus consists of 79 protons (red) and 118 neutrons (blue). Atomic structure of gold includes atomic number, atomic weight, electron configuration 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Each orbital can hold a certain amount of electrons; It has a melting point of 1064 degrees celsius. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). 2, 8, 18, 32, 18, 1. These electrons will exist in determined orbitals around the nucleus.. Atomic structure of gold includes atomic number, atomic weight, electron configuration

Atomic structure of gold includes atomic number, atomic weight, electron configuration. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings).
2, 8, 18, 32, 18, 1. It has a melting point of 1064 degrees celsius. 79), the most common isotope of this element.

Atomic structure of gold includes atomic number, atomic weight, electron configuration 79 electrons (white) successively occupy available electron shells (rings). The nucleus consists of 79 protons (red) and 118 neutrons (orange). Atomic structure of gold includes atomic number, atomic weight, electron configuration It has a melting point of 1064 degrees celsius. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1. 79), the most common isotope of this element. The nucleus consists of 79 protons (red) and 118 neutrons (blue). The nucleus consists of 79 protons (red) and 118 neutrons (blue).

79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... These electrons will exist in determined orbitals around the nucleus. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. The nucleus consists of 79 protons (red) and 118 neutrons (blue).
79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. Each orbital can hold a certain amount of electrons; It has a melting point of 1064 degrees celsius. 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). The nucleus consists of 79 protons (red) and 118 neutrons (blue).. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

Each orbital can hold a certain amount of electrons; It has a melting point of 1064 degrees celsius. 2, 8, 18, 32, 18, 1... It has a melting point of 1064 degrees celsius.

Atomic structure of gold includes atomic number, atomic weight, electron configuration It has a melting point of 1064 degrees celsius. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Each orbital can hold a certain amount of electrons; These electrons will exist in determined orbitals around the nucleus. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). It has a melting point of 1064 degrees celsius.. Each orbital can hold a certain amount of electrons;
79 electrons (white) successively occupy available electron shells (rings).. These electrons will exist in determined orbitals around the nucleus.

Atomic structure of gold includes atomic number, atomic weight, electron configuration Atomic structure of gold includes atomic number, atomic weight, electron configuration 2, 8, 18, 32, 18, 1. These electrons will exist in determined orbitals around the nucleus. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 79), the most common isotope of this element. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.. And for gold, each energy level will hold:

2, 8, 18, 32, 18, 1... . Each orbital can hold a certain amount of electrons;

79 electrons (white) successively occupy available electron shells (rings). 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

79), the most common isotope of this element. And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. It has a melting point of 1064 degrees celsius. 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons; These electrons will exist in determined orbitals around the nucleus. 79), the most common isotope of this element. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings)... 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

Atomic structure of gold includes atomic number, atomic weight, electron configuration. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 79 electrons (white) successively occupy available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. The nucleus consists of 79 protons (red) and 118 neutrons (orange). These electrons will exist in determined orbitals around the nucleus.. The nucleus consists of 79 protons (red) and 118 neutrons (blue).

These electrons will exist in determined orbitals around the nucleus. And for gold, each energy level will hold: 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). It has a melting point of 1064 degrees celsius. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79), the most common isotope of this element. 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus.. It has a melting point of 1064 degrees celsius.

79), the most common isotope of this element... 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus... 79), the most common isotope of this element.

It has a melting point of 1064 degrees celsius.. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Atomic structure of gold includes atomic number, atomic weight, electron configuration

79 electrons (white) successively occupy available electron shells (rings).. And for gold, each energy level will hold: 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. It has a melting point of 1064 degrees celsius. The nucleus consists of 79 protons (red) and 118 neutrons (blue). Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus.. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

79), the most common isotope of this element. Each orbital can hold a certain amount of electrons;.. Each orbital can hold a certain amount of electrons;

26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). 79 electrons (white) successively occupy available electron shells (rings). Each orbital can hold a certain amount of electrons;. 2, 8, 18, 32, 18, 1.

Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (blue). These electrons will exist in determined orbitals around the nucleus. It has a melting point of 1064 degrees celsius. And for gold, each energy level will hold: The nucleus consists of 79 protons (red) and 118 neutrons (orange). Atomic structure of gold includes atomic number, atomic weight, electron configuration 79 electrons (white) successively occupy available electron shells (rings).

2, 8, 18, 32, 18, 1. Each orbital can hold a certain amount of electrons; It has a melting point of 1064 degrees celsius. The nucleus consists of 79 protons (red) and 118 neutrons (blue). 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1.

79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).. 79 electrons (white) successively occupy available electron shells (rings). 2, 8, 18, 32, 18, 1. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus... These electrons will exist in determined orbitals around the nucleus.

And for gold, each energy level will hold: 79 electrons (white) successively occupy available electron shells (rings). 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). And for gold, each energy level will hold: 79), the most common isotope of this element. Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (blue). The nucleus consists of 79 protons (red) and 118 neutrons (orange). It has a melting point of 1064 degrees celsius.

79 electrons (white) successively occupy available electron shells (rings).. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Atomic structure of gold includes atomic number, atomic weight, electron configuration Each orbital can hold a certain amount of electrons; The nucleus consists of 79 protons (red) and 118 neutrons (blue). 2, 8, 18, 32, 18, 1. The nucleus consists of 79 protons (red) and 118 neutrons (orange). And for gold, each energy level will hold: These electrons will exist in determined orbitals around the nucleus. It has a melting point of 1064 degrees celsius. 79), the most common isotope of this element.. The nucleus consists of 79 protons (red) and 118 neutrons (orange).

The nucleus consists of 79 protons (red) and 118 neutrons (orange)... Atomic structure of gold includes atomic number, atomic weight, electron configuration 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79 electrons (white) successively occupy available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. Atomic structure of gold includes atomic number, atomic weight, electron configuration

79 electrons (white) successively occupy available electron shells (rings). It has a melting point of 1064 degrees celsius. 2, 8, 18, 32, 18, 1. 79 electrons (white) successively occupy available electron shells (rings). 79 electrons (white) successively occupy available electron shells (rings).

79), the most common isotope of this element. 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Each orbital can hold a certain amount of electrons; 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus.

These electrons will exist in determined orbitals around the nucleus... Each orbital can hold a certain amount of electrons; Atomic structure of gold includes atomic number, atomic weight, electron configuration It has a melting point of 1064 degrees celsius. 26.03.2020 · the gold atomic structure has 79 protons and 118 neutrons in its nucleus. 79), the most common isotope of this element. 2, 8, 18, 32, 18, 1.. It has a melting point of 1064 degrees celsius.

The nucleus consists of 79 protons (red) and 118 neutrons (blue).. The nucleus consists of 79 protons (red) and 118 neutrons (blue). 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). Atomic structure of gold includes atomic number, atomic weight, electron configuration These electrons will exist in determined orbitals around the nucleus. And for gold, each energy level will hold: 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).

These electrons will exist in determined orbitals around the nucleus... 79), the most common isotope of this element. It has a melting point of 1064 degrees celsius. The nucleus consists of 79 protons (red) and 118 neutrons (blue). 79 electrons (green) bind to the nucleus, successively occupying available electron shells (rings). These electrons will exist in determined orbitals around the nucleus. Atomic structure of gold includes atomic number, atomic weight, electron configuration And for gold, each energy level will hold: Each orbital can hold a certain amount of electrons;. Atomic structure of gold includes atomic number, atomic weight, electron configuration
